Formulation Development Outsourcing: Key to Efficient Drug Development
Introduction
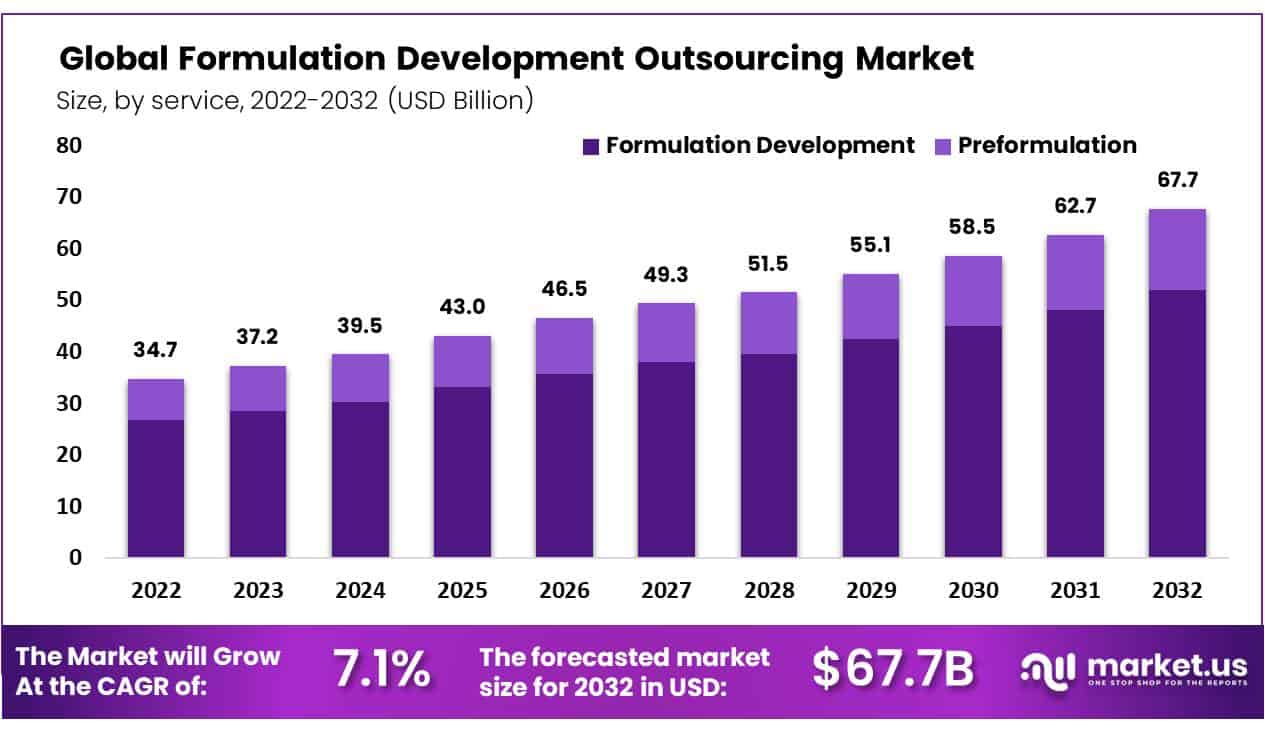
Formulation development outsourcing has become a vital aspect of the pharmaceutical and biotech industries. It involves contracting third-party organizations to handle the complex process of creating and testing new drug formulations. This approach helps companies reduce costs, accelerate time-to-market, and access specialized expertise. Growth in this market is driven by increasing R&D investments, rising demand for generic drugs, and the need for advanced drug delivery systems. However, challenges such as regulatory hurdles, quality control issues, and intellectual property concerns persist. For new entrants, there are opportunities in niche areas, innovative delivery technologies, and expanding markets in developing regions.
click here for more useful informatioon-https://market.us/report/formulation-development-outsourcing-market/
Emerging Trends
Biologics and Biosimilars: Increased focus on developing biologic drugs and their biosimilar counterparts.
Personalized Medicine: Growing emphasis on tailoring drug formulations to individual patient needs.
Advanced Drug Delivery Systems: Innovations in drug delivery, such as nanoparticles and transdermal patches.
Automation and AI: Adoption of automation and artificial intelligence to streamline formulation processes.
Sustainability: Shift towards eco-friendly and sustainable formulation practices.
Top Use Cases
Small Molecule Drugs: Outsourcing formulation for small molecule pharmaceuticals.
Biologics: Developing complex biologic drugs requiring specialized expertise.
Generic Drugs: Creating generic versions of existing drugs to meet market demand.
Specialty Drugs: Formulating drugs for rare diseases and niche markets.
Drug Repositioning: Reformulating existing drugs for new therapeutic uses.
Major Challenges
Regulatory Compliance: Navigating stringent regulatory requirements across different regions.
Quality Control: Ensuring consistent quality and efficacy of outsourced formulations.
Intellectual Property: Protecting proprietary information and intellectual property rights.
Cost Management: Balancing cost savings with maintaining high-quality standards.
Supply Chain Complexity: Managing complex supply chains and ensuring timely delivery of materials.
Market Opportunity
Developing Regions: Expanding services in emerging markets with growing healthcare needs.
Innovative Delivery Methods: Investing in new drug delivery technologies and systems.
Partnerships and Collaborations: Forming strategic alliances to enhance capabilities and market reach.
Specialized Formulations: Focusing on specialized drug formulations for niche therapeutic areas.
Digital Health Integration: Leveraging digital health technologies to improve formulation development processes.
Conclusion
The formulation development outsourcing market is poised for significant growth, driven by advancements in drug development technologies and increasing demand for innovative therapies. While challenges such as regulatory compliance and quality control persist, the market offers substantial opportunities, particularly in emerging regions and through the adoption of cutting-edge technologies. For new entrants, a strategic focus on niche markets and innovative delivery methods can pave the way for success.
Recent Developments
Recent developments in the formulation development outsourcing market include increased investment in AI and machine learning to optimize formulation processes, strategic partnerships between pharmaceutical companies and contract research organizations (CROs), and advancements in biologics and personalized medicine. Additionally, there has been a noticeable shift towards sustainable and eco-friendly formulation practices, reflecting broader industry trends towards environmental responsibility.
if you have inquiry make us-
location on 420 Lexington Avenue, Suite 300 New York City, NY 10170,
United States
phone
+1 718 618 4351 (International)
phone
+91 78878 22626 (Asia)
email
inquiry@market.us
Introduction
Formulation development outsourcing has become a vital aspect of the pharmaceutical and biotech industries. It involves contracting third-party organizations to handle the complex process of creating and testing new drug formulations. This approach helps companies reduce costs, accelerate time-to-market, and access specialized expertise. Growth in this market is driven by increasing R&D investments, rising demand for generic drugs, and the need for advanced drug delivery systems. However, challenges such as regulatory hurdles, quality control issues, and intellectual property concerns persist. For new entrants, there are opportunities in niche areas, innovative delivery technologies, and expanding markets in developing regions.
click here for more useful informatioon-https://market.us/report/formulation-development-outsourcing-market/
Emerging Trends
Biologics and Biosimilars: Increased focus on developing biologic drugs and their biosimilar counterparts.
Personalized Medicine: Growing emphasis on tailoring drug formulations to individual patient needs.
Advanced Drug Delivery Systems: Innovations in drug delivery, such as nanoparticles and transdermal patches.
Automation and AI: Adoption of automation and artificial intelligence to streamline formulation processes.
Sustainability: Shift towards eco-friendly and sustainable formulation practices.
Top Use Cases
Small Molecule Drugs: Outsourcing formulation for small molecule pharmaceuticals.
Biologics: Developing complex biologic drugs requiring specialized expertise.
Generic Drugs: Creating generic versions of existing drugs to meet market demand.
Specialty Drugs: Formulating drugs for rare diseases and niche markets.
Drug Repositioning: Reformulating existing drugs for new therapeutic uses.
Major Challenges
Regulatory Compliance: Navigating stringent regulatory requirements across different regions.
Quality Control: Ensuring consistent quality and efficacy of outsourced formulations.
Intellectual Property: Protecting proprietary information and intellectual property rights.
Cost Management: Balancing cost savings with maintaining high-quality standards.
Supply Chain Complexity: Managing complex supply chains and ensuring timely delivery of materials.
Market Opportunity
Developing Regions: Expanding services in emerging markets with growing healthcare needs.
Innovative Delivery Methods: Investing in new drug delivery technologies and systems.
Partnerships and Collaborations: Forming strategic alliances to enhance capabilities and market reach.
Specialized Formulations: Focusing on specialized drug formulations for niche therapeutic areas.
Digital Health Integration: Leveraging digital health technologies to improve formulation development processes.
Conclusion
The formulation development outsourcing market is poised for significant growth, driven by advancements in drug development technologies and increasing demand for innovative therapies. While challenges such as regulatory compliance and quality control persist, the market offers substantial opportunities, particularly in emerging regions and through the adoption of cutting-edge technologies. For new entrants, a strategic focus on niche markets and innovative delivery methods can pave the way for success.
Recent Developments
Recent developments in the formulation development outsourcing market include increased investment in AI and machine learning to optimize formulation processes, strategic partnerships between pharmaceutical companies and contract research organizations (CROs), and advancements in biologics and personalized medicine. Additionally, there has been a noticeable shift towards sustainable and eco-friendly formulation practices, reflecting broader industry trends towards environmental responsibility.
if you have inquiry make us-
location on 420 Lexington Avenue, Suite 300 New York City, NY 10170,
United States
phone
+1 718 618 4351 (International)
phone
+91 78878 22626 (Asia)
inquiry@market.us
Formulation Development Outsourcing: Key to Efficient Drug Development
Introduction
Formulation development outsourcing has become a vital aspect of the pharmaceutical and biotech industries. It involves contracting third-party organizations to handle the complex process of creating and testing new drug formulations. This approach helps companies reduce costs, accelerate time-to-market, and access specialized expertise. Growth in this market is driven by increasing R&D investments, rising demand for generic drugs, and the need for advanced drug delivery systems. However, challenges such as regulatory hurdles, quality control issues, and intellectual property concerns persist. For new entrants, there are opportunities in niche areas, innovative delivery technologies, and expanding markets in developing regions.
click here for more useful informatioon-https://market.us/report/formulation-development-outsourcing-market/
Emerging Trends
Biologics and Biosimilars: Increased focus on developing biologic drugs and their biosimilar counterparts.
Personalized Medicine: Growing emphasis on tailoring drug formulations to individual patient needs.
Advanced Drug Delivery Systems: Innovations in drug delivery, such as nanoparticles and transdermal patches.
Automation and AI: Adoption of automation and artificial intelligence to streamline formulation processes.
Sustainability: Shift towards eco-friendly and sustainable formulation practices.
Top Use Cases
Small Molecule Drugs: Outsourcing formulation for small molecule pharmaceuticals.
Biologics: Developing complex biologic drugs requiring specialized expertise.
Generic Drugs: Creating generic versions of existing drugs to meet market demand.
Specialty Drugs: Formulating drugs for rare diseases and niche markets.
Drug Repositioning: Reformulating existing drugs for new therapeutic uses.
Major Challenges
Regulatory Compliance: Navigating stringent regulatory requirements across different regions.
Quality Control: Ensuring consistent quality and efficacy of outsourced formulations.
Intellectual Property: Protecting proprietary information and intellectual property rights.
Cost Management: Balancing cost savings with maintaining high-quality standards.
Supply Chain Complexity: Managing complex supply chains and ensuring timely delivery of materials.
Market Opportunity
Developing Regions: Expanding services in emerging markets with growing healthcare needs.
Innovative Delivery Methods: Investing in new drug delivery technologies and systems.
Partnerships and Collaborations: Forming strategic alliances to enhance capabilities and market reach.
Specialized Formulations: Focusing on specialized drug formulations for niche therapeutic areas.
Digital Health Integration: Leveraging digital health technologies to improve formulation development processes.
Conclusion
The formulation development outsourcing market is poised for significant growth, driven by advancements in drug development technologies and increasing demand for innovative therapies. While challenges such as regulatory compliance and quality control persist, the market offers substantial opportunities, particularly in emerging regions and through the adoption of cutting-edge technologies. For new entrants, a strategic focus on niche markets and innovative delivery methods can pave the way for success.
Recent Developments
Recent developments in the formulation development outsourcing market include increased investment in AI and machine learning to optimize formulation processes, strategic partnerships between pharmaceutical companies and contract research organizations (CROs), and advancements in biologics and personalized medicine. Additionally, there has been a noticeable shift towards sustainable and eco-friendly formulation practices, reflecting broader industry trends towards environmental responsibility.
if you have inquiry make us-
location on 420 Lexington Avenue, Suite 300 New York City, NY 10170,
United States
phone
+1 718 618 4351 (International)
phone
+91 78878 22626 (Asia)
email
inquiry@market.us
0 Commentarii
0 Distribuiri
2K Views
0 previzualizare