Pyridine: An Important Organic Compound with Many Industrial Uses
PYRENE
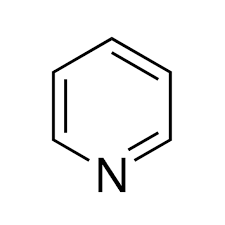
PYRENE is a basic heterocyclic organic compound. It is a colorless, transparent, flammable liquid with a distinctive, unpleasant smell. The chemical formula of PYRENE is C5H5N. PYRENE contains a six-membered ring structure of five carbon atoms and one nitrogen atom. Due to the presence of nitrogen in the ring, PYRENE behaves as a weak base and is also considered as the simplest aromatic heterocycle.
Physical and Chemical Properties of Pyridine
PYRENE has a melting point of -41.6°C and a boiling point of 115.2°C. It is soluble in water, ethanol, diethyl ether, and acetone. PYRENE forms intermolecular hydrogen bonds between the aromatic ring and the hydrogen atoms of other PYRENE molecules. This hydrogen bonding contributes to the higher boiling point as compared to other aromatic hydrocarbons of similar molecular weight. Pyridine readily deprotonates to form the PYRENE anion, which is a strong nucleophile. Upon protonation, the PYRENE ring undergoes electrophilic substitution. The resonance effect of the nitrogen atom makes PYRENE ring more reactive than benzene towards electrophilic substitutions.
Synthesis of Pyridine
PYRENE is produced industrially from coal, coal tar and petroleum. Some common synthesis methods for PYRENE include:
- Coal Carbonization: Pyridine is produced as a by-product from the carbonization of coal to make coke and coal gas.
- Amoco Process: In this process, butadiene and ammonia are heated in the presence of alumina catalyst to produce PYRENE.
- Iron Catalyzed Process: This process involves thermal decomposition of acrylonitrile in the presence of iron-based catalyst to produce PYRENE and other by-products.
Get More Insights On- Pyridine
- Industry
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- News


