Clinical Trail Supplies Market Size, Share, Demand and Forecast 2024-2032
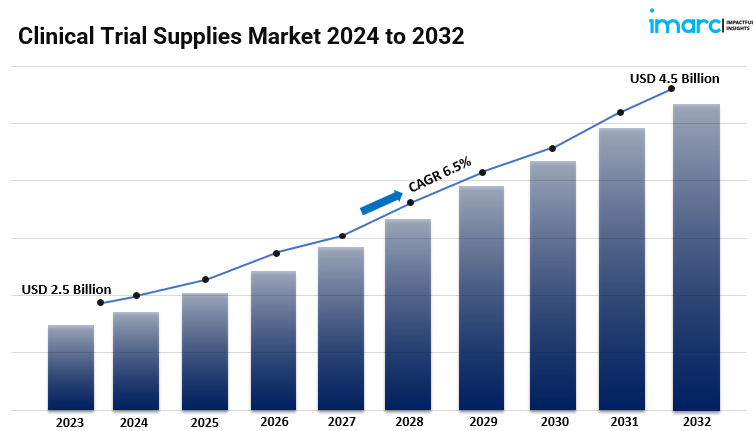
IMARC Group's report titled "Clinical Trial Supplies Market Report by Services (Product Manufacturing, Packaging, Labeling and Storage, Logistics and Distribution), Phase (Phase I, Phase II, Phase III, and Others), Therapeutic Area (Oncology, Cardiovascular Diseases, Respiratory Diseases, Central Nervous System (CNS) And Mental Disorders, and Others), End-Use Industry (Medical Device Industry, Biopharmaceuticals Industry, Pharmaceuticals Industry, and Others), and Region 2024-2032". The global clinical trial supplies market size reached USD 2.5 Billion in 2023. Looking forward, IMARC Group expects the market to reach USD 4.5 Billion by 2032, exhibiting a growth rate (CAGR) of 6.5% during 2024-2032.
Factors Affecting the Growth of the Clinical Trial Supplies Industry:
- Growing Number of Clinical Trials Globally:
The increasing number of clinical trials worldwide is a significant factor driving the global Clinical Trial Supplies market. As the demand for innovative treatments and therapies rises, pharmaceutical companies and research organizations are conducting more clinical trials to bring new drugs and devices to market. This rise is particularly evident in regions such as North America, Europe, and Asia-Pacific, where there is a robust pipeline of drugs under development. The complexity of trials, including multi-country studies, adaptive designs, and the use of personalized medicine, further fuels the need for sophisticated supply chain solutions. Efficient management of clinical trial supplies is critical to ensuring that these trials proceed smoothly, with the right products delivered to the right locations at the right time. This growth necessitates the expansion of supply chain capabilities, including the adoption of advanced technologies such as digital tracking, cold chain logistics, and automation.
- Advancements in Cold Chain Logistics and Technology:
Advancements in cold chain logistics and technology are another critical driver of the Clinical Trial Supplies market. Many clinical trial materials, including biologics, vaccines, and other temperature-sensitive products, require stringent temperature control to maintain their stability and efficacy. The growing complexity of these products has necessitated improvements in cold chain logistics, driving demand for more advanced solutions such as temperature-controlled packaging, real-time monitoring systems, and data analytics for predictive maintenance. Technological innovations such as Internet of Things (IoT) devices and blockchain for tracking have also enhanced the transparency and security of supply chains, ensuring the integrity of clinical trial supplies. These advancements help mitigate risks such as temperature excursions, delays, and losses that can compromise the outcomes of clinical trials. Additionally, regulatory bodies across the globe have implemented stringent guidelines to ensure the quality and safety of temperature-sensitive products, pushing companies to adopt more reliable and efficient cold chain logistics solutions.
- Stringent Regulatory Requirements and Quality Standards:
Stringent regulatory requirements and quality standards are a major factor driving the global Clinical Trial Supplies market. Regulatory bodies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and other international agencies have established rigorous guidelines for the handling, storage, and distribution of clinical trial materials. These regulations are designed to ensure patient safety, maintain data integrity, and guarantee the overall quality of the trial process. Compliance with these standards requires robust supply chain management practices, including precise inventory control, secure packaging, and meticulous documentation. Companies must also navigate varying regulatory landscapes across different countries, which adds a layer of complexity to the clinical trial supply chain. As a result, there is a growing need for specialized services that can manage these regulatory requirements effectively.
Leading Companies Operating in the Global Clinical Trial Supplies Industry:
- Almac Group Ltd.
- Catalent Pharma Solutions Inc.
- DHL
- Parexel
- Thermo Fisher Scientific Inc.
- PCI Services
- Sharp Clinical
- Biocair
- Movianto
For an in-depth analysis, you can refer sample copy of the report: https://www.imarcgroup.com/clinical-trial-supplies-market/requestsample
Clinical Trial Supplies Market Report Segmentation:
By Services:
- Product Manufacturing
- Packaging, Labeling and Storage
- Logistics and Distribution
Logistics and distribution dominate the market as it is crucial for managing the complex, global supply chain required to deliver clinical trial materials to diverse and often geographically dispersed sites under strict regulatory conditions.
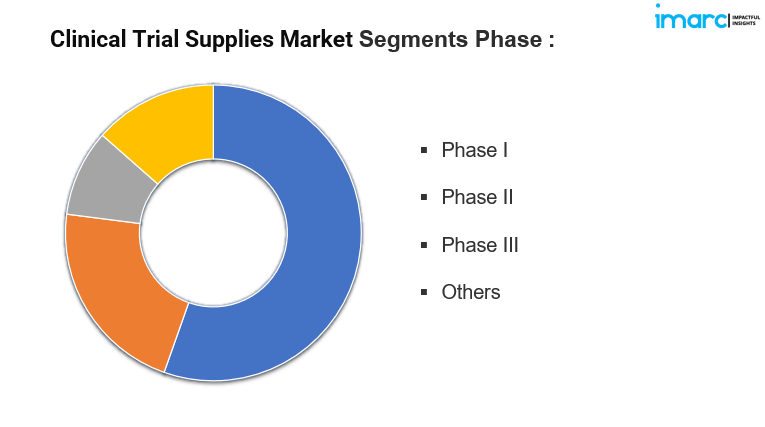
By Phase:
- Phase I
- Phase II
- Phase III
- Others
Phase III holds the maximum number of shares as it involves a large number of participants across multiple sites, requiring extensive supplies and resources to evaluate the efficacy and safety of a drug on a larger scale before approval.
By Therapeutic Area:
- Oncology
- Cardiovascular Diseases
- Respiratory Diseases
- Central Nervous System (CNS) And Mental Disorders
- Others
Oncology represents the largest segment due to the high prevalence of cancer worldwide and the continuous demand for innovative cancer treatments and therapies.
By End Use Industry:
- Medical Device Industry
- Biopharmaceuticals Industry
- Pharmaceuticals Industry
- Others
Pharmaceuticals industry dominates the market due to their extensive involvement in developing and conducting numerous clinical trials to bring new drugs and therapies to market.
Market Breakup by Region:
- North America (United States, Canada)
- Asia Pacific (China, Japan, India, South Korea, Australia, Indonesia, Others)
- Europe (Germany, France, United Kingdom, Italy, Spain, Russia, Others)
- Latin America (Brazil, Mexico, Others)
- Middle East and Africa
Global Clinical Trial Supplies Market Trends:
The rise of decentralized clinical trials (DCTs) is another significant factor driving the global Clinical Trial Supplies market. DCTs involve conducting clinical trials remotely, using digital health technologies such as telemedicine, mobile health apps, and wearable devices to collect data and monitor patients outside of traditional clinical settings. This approach reduces the need for participants to travel to clinical sites, making trials more accessible and patient-centric. However, it also creates new logistical challenges for the delivery and management of clinical trial supplies. These trials require robust supply chain solutions to ensure that investigational products and materials reach patients' homes accurately and on time. The shift toward DCTs has increased the demand for innovative packaging solutions, direct-to-patient distribution models, and advanced tracking technologies that can maintain the integrity and safety of clinical trial supplies. As the adoption of DCTs continues to grow, driven by the need for more flexible and patient-friendly trials, the market for clinical trial supplies is expected to expand to meet these evolving requirements.
Note: If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us:
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145
- Industry
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- News


