Achieving UK Quality Management Certification and UK ISO 13485 Services.

Summary:
- UK Quality Management Certification is the internationally recognized quality management system standard specific to the medical device industry.
- It mandates comprehensive requirements for establishing and maintaining robust QMS processes to ensure quality and regulatory compliance in medical device design, production, and servicing.
- Key components encompass risk management, documentation, design controls, supplier management, and corrective/preventive actions.
- Certification entails a multi-stage audit process by an accredited third-party body, with ongoing surveillance audits mandated.
- For UK ISO 13485 Services, benefits include demonstrating regulatory compliance, improving product safety and quality, enhancing customer trust, streamlining operations, and gaining a competitive edge.
Short Description:
Ensuring compliance with the UK Quality Management Certification standard via comprehensive quality management services is essential for UK ISO 13485 Services. This ensures regulatory adherence, assures product quality, and fosters competitiveness in the market.
Introduction:
In the tightly regulated medical device sector, upholding the highest quality and safety standards is non-negotiable. As regulatory requirements continually evolve, UK ISO 13485 Services face increasing pressure to validate robust quality management processes. They navigate the regulatory landscape overseen by the Medicines and Healthcare Products Regulatory Agency (MHRA). Established to ensure the safety, quality, and efficacy of medical devices, MHRA plays a vital role in regulating the medical device market in the UK. For UK-based services, obtaining UK Quality Management Certification is essential to ensure compliance with regulatory standards and maintain a competitive edge in the market.
The surge of proper regulation of medical devices has resulted in the adoption of the UK Quality Management Certification, recognized as the foremost global standard, that emphasizes its rigorous mandates for establishing an efficient quality management system customized for the medical device sector. Accessing UK ISO 13485 Services has become crucial to meet regulatory benchmarks to sustain in the industry.
Image: https://www.pexels.com/photo/person-holding-document-papers-259006/
The UK Quality Management Certification Standard:
UK Quality Management Certification serves as the internationally harmonized standard defining quality management system requirements for organizations involved in designing, producing, installing, and servicing medical devices. Its comprehensive set of requirements aims to ensure the consistent delivery of safe, effective devices that fulfill customer as well as all applicable regulatory expectations. Fundamentally based on the renowned ISO 9001 standard, UK Quality Management Certification incorporates additional sector-specific criteria crucial for the medical device industry. Acquiring this certification is integral for compliance with these standards. With the assurance of this certification, organizations can demonstrate their commitment to upholding the highest quality and safety standards in the medical device sector, fostering trust and confidence among stakeholders.
Critical Quality Management Elements for UK ISO 13485 Services:
At its essence, UK Quality Management Certification requires a comprehensive, process-oriented approach to quality management throughout every stage of medical device development. UK ISO 13485 Services are essential for navigating these requirements effectively. Vital components of the quality management system (QMS) that UK services must focus on through the standards encompass:
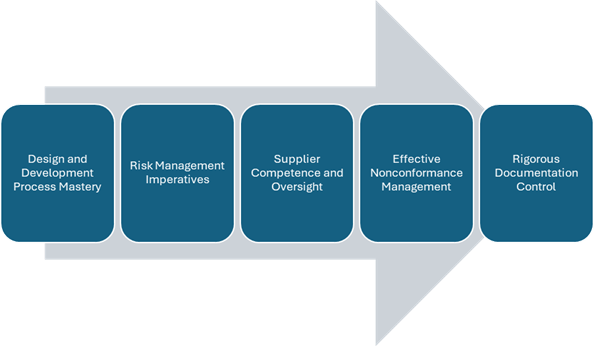
- Design and Development Process Mastery: Strict controls must be enforced over design and development activities to verify devices meet defined user requirements, intended use specifications, and all applicable regulatory mandates.
- Risk Management Imperatives: UK ISO 13485 Services must implement and systematically execute detailed risk management processes to identify, analyze, evaluate, and control potential risks associated with their medical devices throughout the product lifecycle.
- Supplier Competence and Oversight: Criteria must be instituted for evaluating and continually monitoring suppliers’ capabilities to ensure that all procured products and services consistently conform to specified quality requirements.
- Effective Nonconformance Management: Structured processes are required for addressing non-conformances through containment, investigation, root cause analysis, and implementing appropriate corrective and preventive actions to drive continuous improvement.
- Rigorous Documentation Control: Widespread documentation requirements span quality policies, procedures, and records governing every stage from design/development through production, distribution, and post-market surveillance. Robust document control processes are vital.
Path to UK ISO 13485 Services:
UK ISO 13485 Services must undergo a rigorous certification audit conducted by an accredited third-party certification body. This comprehensive audit, conducted in multiple stages, is essential for obtaining UK Quality Management Certification, demonstrating compliance with the necessary regulatory standards, and ensuring the effectiveness of their quality management systems. The typical process includes:

- Application and contractual agreement
- Stage 1 audit - Documentation review against UK Quality Management Certification requirements
- Stage 2 audit - On-site evaluation of implemented QMS processes
- Certification decision and issuance if criteria are fully satisfied
Certification is temporally limited, requiring successful ongoing surveillance audits to provide evidence of continued QMS conformance and effectiveness. Full re-certification audits are also mandated periodically, typically every three years.
Advantages for UK ISO 13485 Services:
Implementing and maintaining UK Quality Management Certification offers a multitude of significant advantages for UK ISO 13485 Services, including:

- Enhanced Product Quality and Safety: UK Quality Management Certification mandates rigorous quality management processes, leading to improved product quality and safety. This fosters confidence among consumers and regulatory authorities in the reliability and effectiveness of medical devices produced by UK ISO 13485 Services.
- Regulatory Compliance: Adhering to UK Quality Management Certification ensures alignment with regulatory requirements, facilitating market access and reducing regulatory hurdles for UK services. Obtaining this certification validates compliance, enhancing credibility and trustworthiness.
- Optimizing Operational Performance: The standard's process-based QMS principles foster streamlined, efficient, and cost-effective operations while catalyzing continuous improvement.
- Competitive Advantage: Services with UK Quality Management Certification gain a competitive edge in the market by demonstrating their commitment to quality and regulatory compliance. Certified services can position themselves as a distinct competitive asset.
- Elevating Customer Confidence: Globally recognized as a benchmark for excellence, UK Quality Management Certification introduces customer confidence regarding product quality and organizational competence.
At CliniExperts, we provide comprehensive UK Quality Management Certification services tailored to support UK ISO 13485 Services in developing a meticulously documented and regulatory-compliant quality management system. With our team of seasoned auditors, we offer extensive expertise in all aspects of the certification process, guiding your organization towards achieving UK Quality Management Certification and fostering a culture of continuous improvement.
Conclusion:
For UK ISO 13485 Services, obtaining UK Quality Management Certification is not just a necessity but a strategic priority in navigating the highly regulated medical device market. This internationally aligned Quality Management System (QMS) standard offers an established structure for ensuring regulatory adherence, managing product risks, and consistently providing safe and effective devices that meet customer needs. While the certification process may pose challenges, the enduring benefits, including enhanced quality assurance, heightened customer trust, and enduring market viability, justify the investment. Engaging in UK Quality Management Certification services is an integral step in this journey toward sustained success.
- Industry
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spellen
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- News


