Breaking Ground: Trends and Developments in the European In Vitro Diagnostics Sector
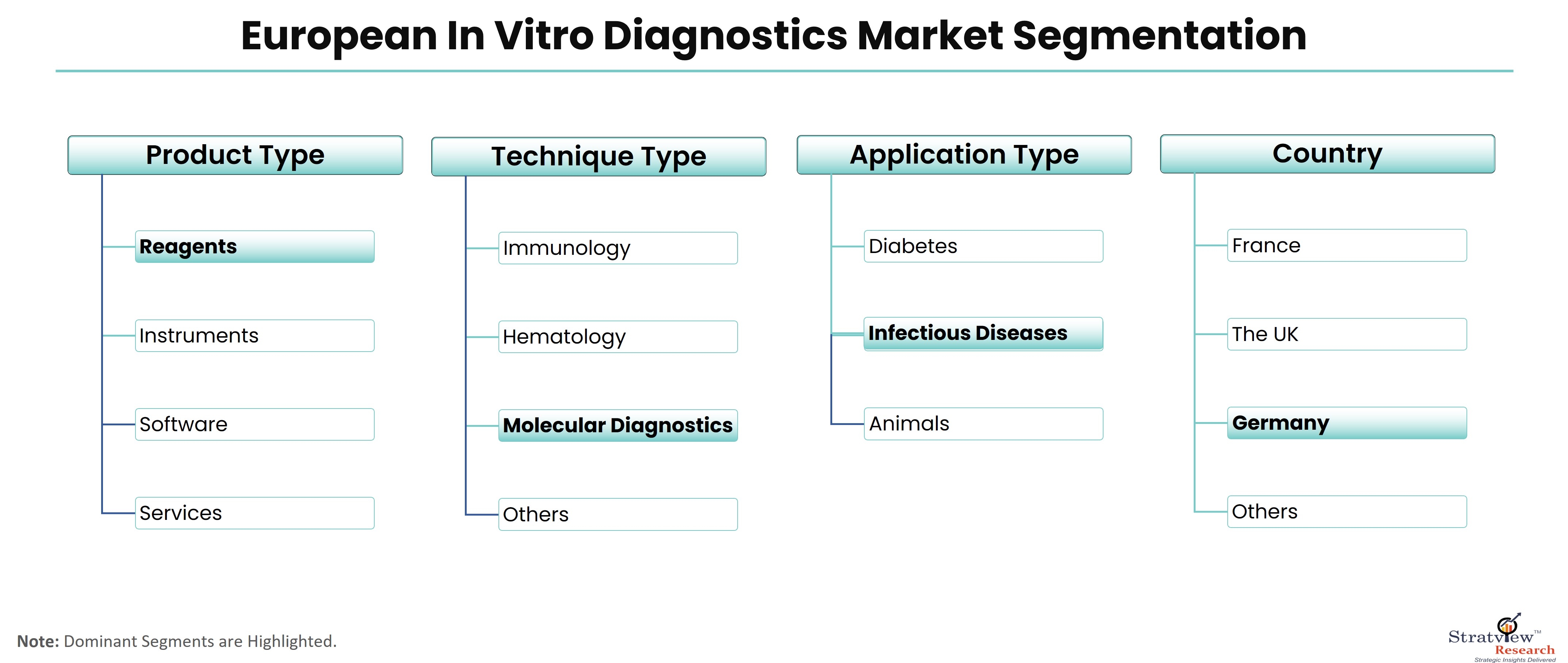
According to Stratview Research, the European in vitro diagnostics market was estimated at USD 15.29 billion in 2022 and is likely to grow at a CAGR of 5.08% during 2023-2028 to reach USD 20.66 billion in 2028.
The European In Vitro Diagnostic (IVD) market is at the forefront of innovation, showcasing a dynamic landscape that spans borders and transcends traditional healthcare boundaries. With a commitment to advancing diagnostic technologies, fostering research, and navigating regulatory landscapes, the European IVD market stands as a beacon of progress. In this article, we explore the interplay of innovation across borders, shaping the dynamic landscape of IVD in Europe.
A Panoramic View of the European IVD Market:
The European IVD market encapsulates a diverse range of diagnostic technologies designed to analyze biological samples, providing crucial information for disease detection, monitoring, and personalized treatment strategies. The collaboration between countries within the European Union (EU) and the collective commitment to advancing healthcare contribute to the dynamic nature of the IVD sector.
Collaboration and Knowledge Exchange:
A hallmark of the European IVD market is the collaboration and knowledge exchange between member countries. Researchers, healthcare professionals, and industry experts across borders engage in collaborative efforts to advance diagnostic technologies, share best practices, and contribute to the collective pool of knowledge.
Regulatory Harmonization:
The European Union's commitment to regulatory harmonization enhances the efficiency and effectiveness of the IVD market. The In Vitro Diagnostic Regulation (IVDR) seeks to standardize regulatory requirements, ensuring a high level of patient safety and fostering an environment conducive to innovation.
Driving Forces of Innovation:
Advanced Molecular Diagnostics:
The European IVD market is witnessing a surge in advanced molecular diagnostics, driven by breakthroughs in genomics and proteomics. The ability to analyze genetic and protein markers at a molecular level enables more precise diagnostics, contributing to personalized medicine and targeted therapies.
Rise of Point-of-Care Testing (POCT):
Point-of-care testing is gaining prominence across Europe, providing rapid and decentralized diagnostic solutions. This trend is particularly impactful in rural or underserved areas, where quick and on-the-spot diagnostics can improve patient outcomes by expediting treatment initiation.
Digital Transformation:
The digital transformation of healthcare is influencing IVD across borders. Digital technologies, including artificial intelligence, data analytics, and cloud-based solutions, are enhancing the interpretation of diagnostic results, streamlining workflows, and contributing to more efficient patient care.
Challenges and Opportunities:
Market Diversity:
The diversity of healthcare systems and market structures across European countries presents challenges for companies operating in the IVD sector. However, this diversity also offers opportunities for customization and tailoring products to specific market needs.
Regulatory Compliance:
Adhering to regulatory standards can be complex given the diversity of regulatory frameworks. However, companies that navigate these challenges successfully gain a competitive advantage, demonstrating their commitment to delivering high-quality, compliant diagnostic solutions.
Conclusion:
Innovation across borders defines the dynamic landscape of the European In Vitro Diagnostic market. The collaborative spirit, regulatory harmonization, and a commitment to advancing diagnostic technologies underscore the region's role as a global leader in healthcare innovation. As the European IVD market continues to evolve, stakeholders must navigate challenges, embrace opportunities, and leverage cross-border collaboration to drive forward the frontiers of diagnostic excellence. The journey towards a healthier and more innovative future is propelled by the collective efforts and shared vision of the European IVD community.
- Industry
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- News


