The Animal Drug Compounding Market to grow on the back of on-demand healthcare, witnessing a CAGR of 8.2%
Persistence Market Research published a report on the animal drug compounding market, which considers the U.S. industry analysis 2014 – 2018 and opportunity assessment 2019 – 2029, and projects that the animal drug compounding market is expected to reach ~ US$ 1 Bn by the end of 2019 in terms of value, and is forecasted to reach ~ US$ 2 Bn by the end of 2029.
Want Insights To US Animal Drug Compounding Market? Ask For Sample! https://www.persistencemarketresearch.com/samples/30058
Company Profiles:
- Hoye’s Pharmacy
- Vertisis Custom Pharmacy
- Smith Caldwell Drug Store
- Sixth Avenue Medical Pharmacy
- Dougherty’s Pharmacy
- Triangle Compounding Pharmacy Inc.
- Medisca Inc.
- Wedgewood Pharmacy
- Millers Pharmacy
Planning To Conclude Your Strategy On A Decisive Note In The US Animal Drug Compounding Market? Glance Through The “Methodology” Implied! https://www.persistencemarketresearch.com/methodology/30058
Availability of Alternative Dosage Forms Boosting the Demand for Animal Drug Compounding
Commercially available veterinary drugs often come in a single dosage or strength. Animal drug compounding is able to produce desired drugs with alternate dosage and varied dosage forms, which is not possible during the production of branded drugs. Such types of animal drug compounding involve concentrating the drug in the flavoured gel that is generally applied to the paw or fur of the animal, and is administered by licking. Also, dosage forms such as transdermal drug delivery in animals, are widely accepted in diverse range of animal drug compounding. To cite an example, anti-parasitic drugs for cattle and anti-flea drugs for dogs and cats are most successful transdermal drug delivery transdermal compounded drug products.
Lower Availability of Generic Substitutes for Veterinary Drugs
Availability of generic substitutes is much lower for veterinary drugs. Most drugs available for animals for critical diseases are branded drugs. Additionally, currently there is no reimbursement available for veterinary drugs, which indicates that most of the drug expense goes out of the pockets of animal owners. In such circumstances, most pet owners prefer resorting to animal drug compounding that are easily available, cheaper than branded equivalents, and do not require multiple prescriptions.
CNS Drugs to be Most Frequently Dispensed Extemporaneous Medications from Animal Drug Compounding Pharmacies
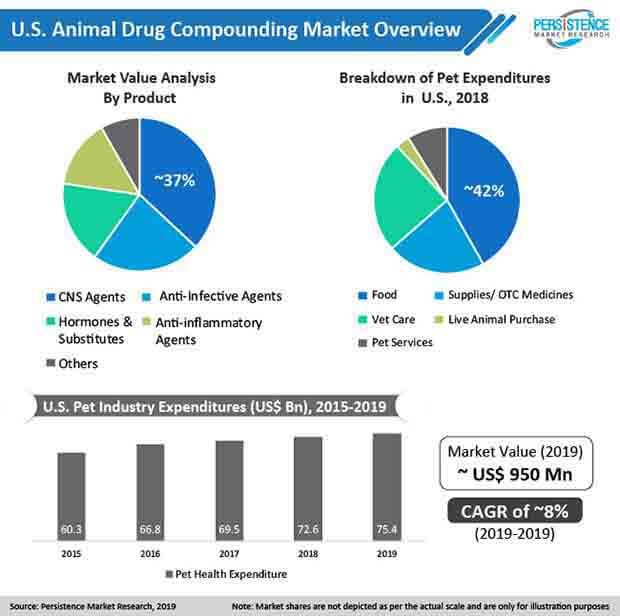
Central nervous system inflammation is the most common cause of neurological disorders in dogs and cats. Abnormal limb coordination, seizures, behavioral changes, and blindness are cited as the most common abnormalities in dogs and cats with central nervous system inflammation. For instance, according to the International Journal of Pharmaceutical Compounding, central nervous system drugs were the most commonly dispensed medications from animal drug compounding pharmacies with approximately 39% of the total veterinary prescriptions.
Absence of Adverse Event Reporting in Animal Drug Compounding
Individual boards of animal drug compounding pharmacies of various states in the U.S. haven’t reported any incidences of adverse events related to animal drug compounding. Furthermore, the U.S. FDA’s adverse drug event reporting system for animals is also not aggregated or comprehensive, and is currently being revised. Since 2001, only 62 compound-related adverse events in animal drug compounding have been reported to the FDA. No definitive trends can be derived out of them. Therefore, absence of adverse event reporting in animal drug compounding will upsurge the demand for animal drug compounding pharmacies.
Buy Full Report Now and Get Upto 20% Discount @ https://www.persistencemarketresearch.com/checkout/30058
Unsafe Compounding Practices May Deter the Growth of Animal Drug Compounding
The primary factor restraining the usage of animal drug compounding is unsafe compounding practices, which include compounding contaminations and non-adherence to GMP regulations. This is very prominent across sterile compounding involving injectable drugs and intravenous solutions. Contaminated sterile environments lead to spread of pathogens and fungal infections, and have resulted in various hazards and drug-related deaths, thus dampening the prospects for animal drug compounding. To avoid unsafe animal drug compounding practices, the U.S. FDA has made it mandatory for all outsourcing facilities manufacturing bulk drugs to be inspected and to secure cGMP certification.
About Us: Persistence Market Research
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales – sales@persistencemarketresearch.com
- Industry
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness
- News


